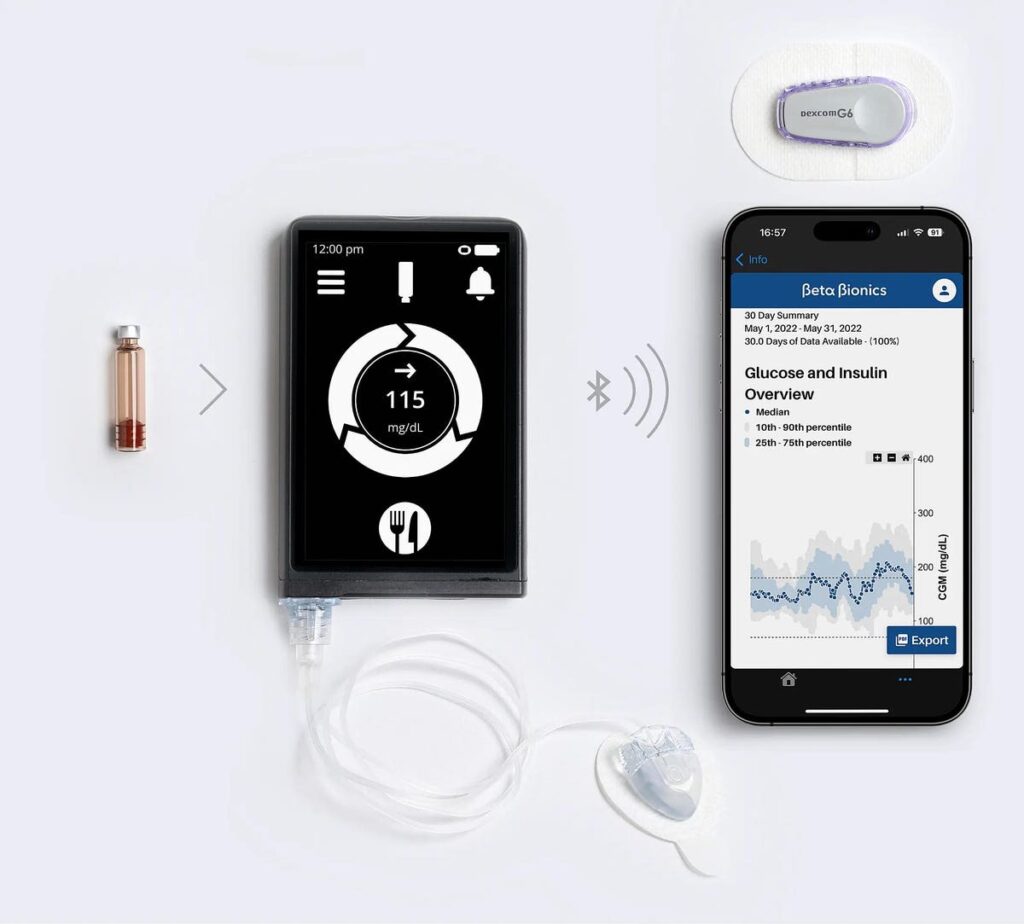
Bionic Pancreas Automated Insulin Pump
The following is the third part of a series on brain-machine integration and biomechanical solutions to restore function to tissues damaged by disease, trauma, or time.
The FDA recently granted clearance to a new insulin pump delivery system, marking a major step forward for those suffering from diabetes. In 2022, the Diabetes Research Institute estimated that over 37 million people in the United States have diabetes or more than 1 in 10 Americans. The CDC estimates that by 2040, over half a billion will be diabetic worldwide.
Not only is it one of the most common chronic diseases in the United States, but it is also among the more cumbersome. Diabetics require regular insulin injections to regulate blood sugar, a hormone most people produce naturally. Insulin injections are costly and time-sensitive, making diabetes an invasive social and financial disease.
Thankfully, new technologies for diabetes are constantly in development. Most recently, insulin pumps have been a popular replacement for regular injections among those with the disease. Rather than single-dose manual injections, an insulin pump delivers insulin doses at specific times throughout the day. These devices can be permanently or temporarily applied to the wearer, improving flexibility and convenience.
In recent weeks, the FDA approved a new insulin pump that takes convenience a step further. The Beta Bionics iLet ACE Pump, in conjunction with the iLet Dosing Decision Software, acts as a bionic pancreas for the diabetic. The role of the pancreas is to automatically produce the right amount of insulin in response to glucose levels in the blood. The bionic pancreas would perform a similar role, delivering a constant stream of insulin automatically adjusted to the wearer’s meals, body type, and long-term health habits.
Whereas the standard insulin pump must be adjusted manually based on what the wearer estimates their carbohydrate count to be, the bionic pancreas adjusts insulin levels internally, requiring the wearer to only input whether their meal was low, medium, or high in carbohydrates. Over time, the artificial pancreas self-adjusts to the user.
The FDA’s approval came following the release of trial results for the bionic pancreas in type 1 diabetics. The study by the Bionic Pancreas Research Group followed 219 type 1 diabetics from the ages of 6 to 79 over a 13-week period.
The analysts used glycated hemoglobin levels as their primary endpoint. This is the level of glucose in a person’s blood. Most diabetics are diagnosed when their glycated hemoglobin levels are above 6.5%. For the Type 1 diabetic, staying as close to this percentage as possible is the goal when delivering insulin, and the higher the percentage, the more dangerous to their general well-being.
FIGURE 1: Mean Glucose Levels According to Time of Day and the Cumulative Distribution of Time in … [+]
The 219 participants using the bionic pancreas over a 13-week span, on average, had their glycated hemoglobin level decreased from 7.9% to 7.3%. The control group’s level remained the same at 7.7%. Dangerous glucose spikes (>54 mg per deciliter) did not differ between control and bionic pancreas participants, and there were no episodes of diabetic ketoacidosis in either group.
While cost remains a major impediment, especially in the United States, where the average dose of insulin costs over $100, the bionic pancreas would greatly reduce the social and time stress of dealing with insulin injections. The only requirement for the user is the few times daily input of carbohydrate estimation. If the price of the bionic pancreas is reasonable, we look forward to the regular implementation of this technology to soon improve the lives of those that need it.