OPA CEO and cofounder Jedd Lewis (far left) and founder Dr. Seb Giwa (far right), with cofounders … [+]
While aging biologists and machine learning scientists are hard at work developing biological aging clocks and ways to reverse them, their breakthroughs might not come in time for many of us. That’s why many high-profile scientists are venturing into an alternative field – halting the degenerative processes using various converging technologies in cryobiology. Most clocks stop ticking at very low temperatures. And some of these technologies are closer to reality than you may think. In fact, many of the people born via in vitro fertilization (IVF), were once cryopreserved as embryos. And the recent progress in organ cryopreservation is staggering.
In its cover story the current issue of Science discusses a deluge of unprecedented organ cryopreservation advances, some of which were announced recently at two of
the largest annual conferences in the field of organ transplantation: the meeting of the Association of Organ Procurement Organizations, which celebrated its 40th anniversary this year, and the American Transplant Congress.
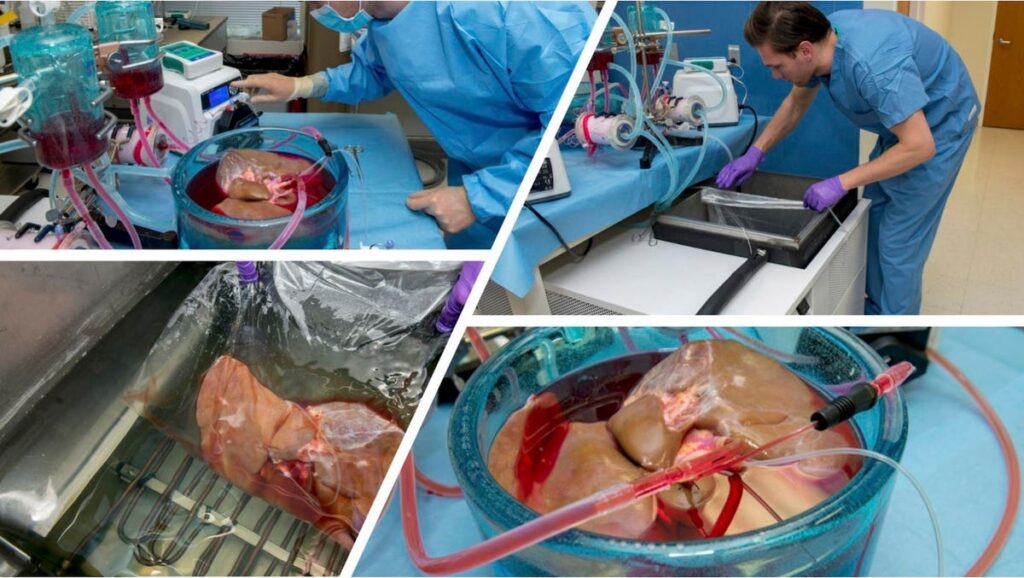
At the American Transplant Congress, researchers from Harvard Medical School, Massachusetts General Hospital, and the biotechnology company Sylvatica Biotech reported the first human livers to be stored at below-freezing temperatures for over 3 days – roughly 10 times the clinically acceptable preservation period using conventional methods1 . The report follows the same research group’s recent study in Nature Biotechnology reporting the first successful storage of human livers at below-freezing temperatures, which was widely covered by publications such as JAMA and The Atlantic and hailed by the head of the U.S. National Institutes of Health as a significant step forward in the field.
Experiments storing human livers at below-freezing temperatures at Harvard’s Massachusetts General … [+]
Meanwhile a research group at Johns Hopkins working with Sylvatica Biotech reported the successful transplantation of mammalian limbs after 3 days of storage at below-freezing temperatures2. If the limb preservation approach can be scaled to humans like the liver preservation approach has been, it could help unblock the stymied practice of limb transplantation. Finding a close match between donors and recipients can be a far more extreme challenge for limb, hand, or facial transplantation than for internal organs. With the ability to send such transplants across a continent or across the globe, orders of magnitude more of these transplants could be feasible.
Later at the Association of Organ Procurement Organizations meeting, in an opening plenary session by Sylvatica Biotech’s CEO and the Director of the National Science Foundation’s ATP-Bio cryopreservation program, the first successful procedure to store mammalian transplant organs at deep cryogenic temperatures was announced. It had been published in Nature Communications only days before. The publication opens the door to storing organs for transplantation the same way that eggs and embryos are stored for in vitro fertilization today. For demonstration purposes, kidneys in the study had been held at deep cryogenic temperatures (below -140℃) for up to 100 days before being warmed to body temperature and transplanted into rats. This study is revolutionary and I hope to cover it in a separate article.
At these temperatures the organs could theoretically be stored for years and even many centuries without any measurable changes at the molecular level. The organs were frozen in time. This is a watershed moment in the field of cryopreservation, and in future decades it may be remembered as the pivotal demonstration that organs can be stored outside of the body for an unlimited duration.
In recent issues I have covered the emerging renaissance in cryopreservation and the work of Dr. Dayong Gao’s group. This month’s announcements may be the clearest sign yet that the field has come of age.
To understand how this came about I delved into the career of the founder and CEO of Sylvatica Biotech, Dr. Sebastian Giwa (called “Seb” by his friends). Seb is credited by many as the chief architect of the surge of scientific interest in cryopreservation over the last several years.
Sebastian Giwa, PhD, MBA
Seb boasts an incredibly long C.V. for someone who has only been working in this area for a decade. Among other entrepreneurial successes he is the founder of several different companies in space that have all grown and prospered, including the nonprofit Organ Preservation Alliance and the public benefit companies Ossium Health and Sylvatica Biotech. Seb is also a co-founder of other major initiatives, including the Biostasis Research Institute and a new branch of the American Society of Transplantation, and sits on the board of directors at NDN (Nevada Donor Network), one of the most successful U.S. organizations responsible for managing organ transplant donation. Seb is the founding Chairman of Expanse Bio and of ATP-Bio Partners, the industry consortium at the U.S. National Science Foundation’s nationwide cryopreservation research program. Recently he was on a short list of biotech leaders (alongside others like Bill Gates, George Church, and J. Craig Venter) tapped by the preeminent scientific journal Nature Biotechnology for its 25th anniversary to describe the areas in which the life sciences will make the biggest impact on society in the coming years.
As it turns out the cryopreservation Renaissance is no accident; it’s been a decade in the making. And according to Seb and others I spoke to, cryopreservation is about to change organ transplantation forever. After that? All of human health.
Can Cryopreservation Solve the Organ Shortage?
On the opening morning of the 40th annual meeting of the Association of Organ Procurement Organizations (“AOPO”) Seb led a plenary session on organ cryopreservation. The new achievements were presented to a packed auditorium, but this was not the usual crowd for a scientific talk: instead of researchers the audience was composed mainly of executives at organ procurement organizations (“OPOs” for short). These nonprofit organizations are tasked by the U.S. government with coordinating all organ donations within their respective geographic regions (typically the size of a U.S. state), working with local hospitals to identify potential donors and then offering those donations to patients on the national transplant waitlist.
For the last several years some OPOs have been heavily engaged in efforts to increase organ cryopreservation funding – including helping persuade the U.S. National Science Foundation to establish a nationwide research program in cryopreservation. An unusual aspect of the kidney cryopreservation breakthrough being presented at AOPO was that five of these OPOs had personally funded the study, matching grants from U.S. science agencies. Several OPOs had also been providing a steady supply of rejected (untransplanted) donor organs for the cryopreservation studies, helping bridge the gap from animal proof-of-concept to human data much more quickly than would be expected for a typical medical research program.
The OPOs have good reason to be interested in organ cryopreservation. Despite twelve straight years of record-breaking increases in transplants performed, most organ offers by U.S. OPOs are rejected by their nearby transplant centers. For instance, in the U.S. a patient who dies waiting for a new liver has had a median of 6 donor liver offers rejected on their behalf by their transplant program. A median of 16 offers has been rejected for each patient who dies on the kidney waitlist. Most patients never become aware that an offer has been made. Even children in need of a heart transplant, who are among the hardest recipients to match to donors, experience something similar. The vast majority receive an acceptable offer, and the transplant team turns down this offer about 85% of the time. Many of these livers, kidneys, and hearts expire before they are accepted by any transplant centers close enough to take them. The studies suggest that many (if not most) of those organs are actually transplantable and would have benefited patients.
Five U.S. organ procurement organizations pooled funding for cryopreservation research, helping … [+]
This problem was highlighted last year in a report by the National Academies of Science, Engineering, and Medicine (NASEM). The NASEM study was triggered in part by oft-cited claims that the main cause of the organ shortage is a simple lack of supply: a failure of OPOs to identify enough suitable donors and facilitate offers for transplantation. But the NASEM report concluded that the situation is much more complex than that. Within localized times and geographies, OPOs frequently offer more transplantable organs than nearby transplant centers accept.
Organ transplantation may be one of the most profound and meaningful achievements of human civilization to date, but an organ outside the body still shares some straightforward limitations with other things that expire quickly. Trying to donate organs (without cryopreservation) is a lot like trying to donate perishable (non-canned) food: there are certainly a lot of starving people in the world, but good luck finding a recipient for every single ounce before it expires. A growing body of evidence suggests that if these organs could be offered to more patients, and especially to patients at transplant programs farther away from the OPO offering the organ, many of them would be accepted.
A crucial factor is that the population of donor organs is remarkably heterogeneous. Each donor has a unique medical history, and dozens of factors can impact the risks and benefits of transplanting a particular organ into a particular waitlist patient. There is no clear line between the organs that are “transplantable” and those that are not. The pool of organs that could theoretically be transplanted (providing a survival benefit and improving the recipient’s quality of life) is at least several-fold beyond what is transplanted today. But as transplant centers go “deeper” into that pool, transplanting organs from donors with more medical complications, those organs are expected to lead to an increased risk of transplant failure, organ rejection, and other adverse side effects. While most of those transplants still succeed, post-transplant patient life expectancy generally decreases as transplant centers go deeper into the donor pool. This means that for a transplant program, one of the weightiest decisions is whether to accept an organ being offered today or wait and see if a “better” offer will come along before it’s too late.
Each transplant center has a unique endowment of practical experience and judgment about which organ offers to accept for their patients. Their organ acceptance practices are also influenced by each center’s unique circumstances such as the patient populations they serve, logistical constraints, and even regulatory and financial risks. Some transplant centers accept more diverse organ offers than others, and these program-specific differences span all major transplant organs (livers, hearts, lungs, and kidneys). The disparities can be extreme: some centers can accept a much wider range of offers from OPOs without compromising outcomes for their patients.
The variation is even more striking when comparing the U.S. to other countries. For example, while Spain and the U.S. annually compete for the distinction of performing the most deceased donor organ transplants per capita in the world (no other country comes close), in Spain far more of these donations come from donors over age 70. These older organs are often presumed by U.S. transplant centers to be of lower quality.
All donor livers are unique, and accepting or rejecting an offer influences the balance of risks: … [+]
These regional and global disparities contribute heavily to the organ shortage. For instance, a recent study compared the kidney acceptance practices of transplant centers in France to those in the U.S. and used this information to model the number of U.S. kidneys that would have been accepted by the French transplant centers based on donor medical history. They found that 62% of U.S. discard kidneys would have been transplanted in France, leading to over 130,000 aggregate life-years that would have been added over the ten-year study period if the U.S. OPOs had been able to offer those kidneys to transplant programs using French organ acceptance practices.
For decades policy solutions have been proposed to mitigate this problem, but there is a ceiling to their effectiveness. While a patient and family can choose to donate, their hospital can notify the OPO, the OPO can offer organs to patients at transplant centers, and a national waitlist can decide who gets the organ offer first… no one can force a surgeon to accept the organ being offered and transplant it into their patient. Crucially, each transplant program acts as an advocate for the individual patient – meaning that they need to take into account that a “better” organ offer might come along tomorrow. The more donor organs an OPO offers, the more selective the nearby transplant centers are incentivized to be.
The NASEM Report noted that variations in acceptance practices of nearby transplant centers have a direct impact on the performance of OPOs, exacerbating the organ shortage. Other studies have identified this phenomenon for liver, heart, lung, and kidney transplantation. This means that a key determinant of success is the OPOs’ proximity to transplant programs that are willing and able to accept organs with a wider range of medical characteristics. Because organs outside the body expire within hours today, many that are donated too far away from these transplant centers become missed opportunities to save patients.
A number of OPOs are now working with Sylvatica Biotech to solve this Achilles Heel in the U.S. transplant system, developing the liver cryopreservation approach as a way to offer donor livers across a much greater time and distance. The aim: untether the OPO from its geography and local circumstances.
Because of limited preservation windows, today donor livers are allocated by distance. A medically … [+]
To understand what this could mean for the organ shortage I spoke with Jedd Lewis, the CEO of the nonprofit Organ Preservation Alliance. Jedd is also the founding Chair of the American Society of Transplantation’s Recovery and Preservation Community of Practice and a member of Sylvatica’s advisory board. I asked him how many more liver transplants could result from Sylvatica’s technologies. His answer: the factors at play are complex, but under the right conditions the potential is nothing short of thousands of additional lifesaving liver transplants each year from U.S. donors. Considering that today there are fewer than 10,000 U.S. liver transplants per year, the impact on the organ shortage would be profound.
This is a far cry from the outlook for transplantation a decade ago. At that time organ cryopreservation was an obscure sub-field that had mostly been orphaned by both funders and the scientific community. To many, the prospect of unlimited organ preservation was a fantasy.
How did we get here?
An “Apollo Program” in Organ Cryopreservation
In Summer 2012 Seb was one of a tiny fraction of entrepreneurs, scientists, and physicians admitted to the Global Solutions Program on NASA’s Silicon Valley campus (“GSP” for short). The program was part of Singularity University, which had been launched as a collaboration between organizations such as Google, NASA, Genentech, Cisco, and a group of high-profile futurists including Peter Diamandis and Ray Kurzweil, with a mission to “prepare humanity for accelerating technological change.” GSP students were given enviable access to highly regarded scientists and engineers. These experts gave them a crash course in a wide range of exponentially advancing technologies such robotics, nanotechnology, genetic engineering, and artificial intelligence. The students were then asked to find new ways to harness these technologies to solve humanity’s “grand challenges” such as hunger, pollution, and disease. The mandate was audacious, but straightforward: positively impact at least 1 billion people.
After brainstorming hundreds of ideas with his classmates Seb zeroed in on a relatively obscure area of technology that had been covered in the GSP program: cryopreservation. The concept itself was immensely powerful. What if we could store living things, such as organs for transplantation and living tissues for research, the same way we can store food, data, energy, and other resources today? This might cause human health to undergo the same transformation as those other industries had. This had already happened at least twice: the first, cryopreservation of human cell lines in the mid-twentieth century, had enabled much of modern medical research. Then cryopreservation of oocytes and embryos in the 1980s had transformed reproductive health by facilitating artificial fertilization.
Modeling had predicted that off-the-shelf organ replacement would increase the average person’s odds … [+]
Yet cryopreservation wasn’t exactly an “exponentially advancing” technology. While cells and embryos were possible to cryopreserve because of their small size, cryogenic storage of larger tissues and whole organs was often dismissed by experts as impossible. Only a handful of research labs in the world were working on the problem and interest from funding agencies was non-existent. So Seb proposed something that was off the wall even by GSP’s standards: while all of the other students were creating futuristic “deep tech” startup companies, Seb established a science advocacy group in the mold of the American Cancer Society. He persuaded several other GSP students to join him, and the Organ Preservation Alliance (“OPA”) was born. Later, when Singularity University (SU) co-founder Peter Diamandis described seven of the most interesting and exciting companies that had previously come out of SU, he named OPA as one of them.
Seb had an unlikely background to jumpstart a stagnant area of science. He had earned a Ph.D. from the Stockholm School of Economics in Sweden, then immigrated to the U.S. to pursue a career in finance. After graduating in the top 5% of his class at Harvard Business School and being named a Baker Scholar, he went to work as an investor at Bridgewater, one of the most successful hedge funds in history.
In its first few years OPA lacked any formal corporate structure. It was a tight knit network of about a dozen volunteers who considered Seb to be the main founder and de facto CEO. Most were early in their careers and educated at elite universities, with skillsets spanning biosciences, engineering, medicine, law, finance, startup entrepreneurship, business development and public relations. According to other OPA co-founders, the common thread was belief in Seb’s vision for the potential of cryopreservation …and a willingness to volunteer enormous amounts of time to OPA while holding demanding full-time jobs.
Ultimately Seb left his career as an investor to split his time between OPA and other mission-driven ventures related to cryopreservation. Then an OPA volunteer, Jedd Lewis, came to Seb with an offer: he’d leave his Ph.D. studies at Stanford, where he had been conducting cryopreservation research, to help Seb fundraise and turn OPA into a fully-fledged and staffed nonprofit organization. Jedd had recently graduated from Harvard Law School and had been pursuing a second career as a research scientist. He’d joined up well after the NASA program that had birthed OPA, but his aspirations were similar to those of Seb and the others: by any means necessary, help to harness the accelerating nature of technological change to make the largest possible impact on human health.
“We had no real plan for sustainability and no resources, literally a negative balance sheet,” Jedd told me. “But after volunteering with Seb for a year I saw the results he was getting. He did things that I hadn’t thought were possible before. It changed my entire model for how someone could make a difference in an area of science. To this day I think I could have gone decades without learning those things, and I wonder how much less of an impact I would have made if I’d never met Seb. So our path was clear. We both leapt without a safety net.”
Jedd served as Seb’s protégé for a year before succeeding him as CEO of OPA, and Seb remained on OPA’s board of directors for another year afterward. To this day Seb remains an active volunteer, and he has donated hundreds of thousands of dollars to the organization. Jedd credits him with helping make many of OPA’s achievements possible even after his time in OPA’s leadership.
Seb discussing new Organ Preservation Alliance initiatives as one of a small group of invited … [+]
OPA and its advisors argued that a convergence of new technologies had made organ cryopreservation a timely challenge. This thesis seems to have been validated by the achievements announced this month, which were built on relatively recent advances in nanotechnology, imaging, ‘omics, computational modeling, and even surgical technique. Over time, Seb and Jedd assembled a large cadre of scientists, surgeons, physicians, scientific organizations, and other stakeholders who were persuaded that a large-scale research effort in cryopreservation should be a top priority of the scientific community.
OPA organized scientific meetings at the White House, Capitol Hill, the U.S. military’s DARPA program, Harvard, and Stanford, while partnering with major scientific and medical societies to organize events aimed at stimulating interest in organ cryopreservation. It worked with major scientific and medical societies, such as the American Society of Transplantation and Association of Organ Procurement Organizations, to establish new programs supporting organ and tissue cryopreservation. Its work has been widely covered in Forbes, as well as in The Economist, WIRED, BBC, and Scientific American.
OPA partnered with these organizations and other key opinion leaders to publish position papers, including a peer-reviewed article in Nature Biotechnology outlining the need for an organ cryopreservation research effort. The paper was co-authored with all of the major U.S. transplant societies and a star-studded lineup of scientists including Robert Langer, George Church, and Ed Boyden, and even Nobel Prize-winning economist Alvin Roth – whose work has focused on finding new ways to ameliorate the organ shortage. It is currently in the top 1% of the most widely read scientific articles published since its release.
OPA’s work has been heavily focused on bringing funding into the field in order to stimulate organ cryopreservation research. Very early on, OPA persuaded the Department of Defense to back cryopreservation research with an initial $15 million in targeted funding. Later it worked with researchers and stakeholder organizations to help persuade the National Science Foundation to launch its nationwide ATP-Bio cryopreservation program, assembling an industry consortium that included vocal OPO backers. With five of these OPOs, OPA established the Biostasis Research Institute, a private philanthropic fund supporting cryopreservation research – including the unprecedented kidney cryopreservation study published this month. OPA itself won funding from the National Science Foundation, the U.S. Department of Defense, several nonprofits focused on health and longevity, the Pineapple Fund, and private philanthropists including famed investors Peter Thiel and Joe Lonsdale, and Skype co-founder Jaan Tallinn. Along the way, OPA helped researchers sustain a steady pace of investigator-initiated grant applications that leveraged the information and arguments that OPA had assembled on the need for the technologies.
Today it seems that OPA has succeeded in making the case for cryopreservation of organs and tissues. The U.S. government and venture investors alike are now beginning to inject real funding into the blossoming field. Seb estimates that approximately $300 million has been committed in the last few years, with another $150 million in follow-on funding likely from existing sources if the current R&D projects remain on track.
The Ninth Donor Organ
When formulating OPA back in 2012 on the NASA campus, Seb had begun to retrace the steps of past cryopreservation breakthroughs. What kinds of living things could already be cryogenically banked? Embryos, oocytes (eggs), cell lines for research… cord blood… bone marrow. It turned out that human bone marrow could already be cryopreserved and transplanted, at least from living donors.
Donor bone marrow is lifesaving for many kinds of blood cancers and a variety of other blood diseases. Successful bone marrow transplants have been performed since the 1950s, but the challenge is finding a source of bone marrow to transplant – especially since donors and recipients must be matched for genetic factors even more precisely than some organ transplants.
Willing donors sign up for a living donor registry. When a patient finds herself in need of a bone marrow transplant, the registry is checked for matching donors and they’re asked to come in and make a donation. The odds of finding a potential match depend heavily on the ethnic background of the patient in need. In the U.S., white patients have a 79% chance of finding a potential match. Black or African American patients, who have a much smaller pool of closely matching donors, only have a 29% chance of finding a potential match. Even when a possible match is found, the living registry approach is far from a guarantee. Across all ages and ethnicities, about half of patients in need die before receiving a donation from the registry.
One well known illustration is the case of Dr. Nalini Ambady, a Stanford professor who was diagnosed with leukemia. A bone marrow transplant would mostly likely have saved her life, and her family and friends conducted a worldwide search for a donor. They found at least six matches that were close enough to enable the procedure. All backed out after initially agreeing to donate. In 2013, after a year of near-misses, Dr. Ambady died. She was only 54.
Seb stumbled onto a question that had been asked by others in the field before: if bone marrow could be cryogenically banked, why not procure it from deceased organ donors who were already providing hearts, livers, and other organs for transplantation? There are nearly 40,000 such donors worldwide each year, and each could potentially donate enough marrow for multiple patients in need. For many patients, when a transplant was needed the matching bone marrow would already be available in the bone marrow bank.
From the perspective of the deceased organ donor and the OPOs, this would be like providing an opportunity to donate an additional lifesaving organ. Traditionally, each donor can provide up to eight lifesaving vital organs: a heart, two lungs, two kidneys, a liver, intestines, and a pancreas. In essence, bone marrow would be the ninth. And if a donor could provide bone marrow to multiple recipients, this might even double the number of lives that each donor could save.
At the end of 2013 Seb was introduced to Louie Helm, a software engineer and the Deputy Director of the Machine Intelligence Research Institute (“MIRI”). Louie’s day job at MIRI was in the then-nascent field of artificial intelligence (A.I.) safety. Over two decades before the rise of ChatGPT prompted more than 300 leaders in the A.I. field to identify the potential misuse of A.I. as a serious existential risk to humanity, MIRI was publishing strategies to mitigate these risks and develop A.I. responsibly. Louie’s other interests in technology were wide-ranging and similarly forward-looking. After discussions about bone marrow banking with Seb and others, Louie published a provocative and data-filled blog post entitled “We Could Cryopreserve Bone Marrow From Organ Donors – But We Don’t.” Seb and Louie set out to change that.
In Early 2015 Seb and Louie obtained the OssiumHealth.com web domain and formed Ossium, Inc. with Seb as founding CEO, aiming to establish a global bank of bone marrow from deceased organ donors. In 2016 Ossium Health, Inc. was formed to carry the activities forward and Louie moved to an advisory role. To help create Ossium Seb approached two well-known researchers in the field of organ transplantation who had published foundational experiments that had helped lay the groundwork for Ossium: Professor Gerald Brandacher at Johns Hopkins, who had carried out foundational studies recovering bone marrow from deceased organ donors, and Professor David Sachs at Columbia University, Harvard Medical School, and Massachusetts General Hospital, who had carried out bone marrow transplants clinically.
Dr. Sachs was best known for discovering many of the human body’s “HLAs,” which are used by a transplant recipient’s body to determine whether to accept or reject a donor’s organ or bone marrow. Over the previous decades he was responsible for much of the progress in immune tolerance induction (therapies to convince a recipient’s body to accept a donor organ without the need for harmful immunosuppression) and xenotransplantation (genetic engineering of animal organs that can be transplanted into humans). Around the time Seb reached out to him Dr. Sachs was awarded the Medawar Prize for this work – the highest award for contributions to the field of transplantation.
Dr. Sachs had carried out clinical studies that coupled transplantation of a kidney with transplantation of bone marrow from the same donor. Miraculously, this procedure allowed kidney transplant recipients to live free of complications without any immune suppression whatsoever – long considered the holy grail in the field of transplantation. Ossium might be able to make this procedure possible for a vastly greater number of patients.
Shortly afterward Ossium partnered with AlloSource, one of the world’s leading providers of transplant tissues from organ donors, and also recruited two more co-founders: Dr. Erik Woods, a well-known cryobiologist who had worked on methods to bank stem cells similar to those from Ossium’s bone marrow bank, and Kevin Caldwell, a young management consultant at the prestigious firm McKinsey & Co. who had worked on a team that Seb had helped manage at the hedge fund Bridgewater. Erik and Kevin manage the company today.
In the years since Ossium’s formation it has partnered with close to half the OPOs in the U.S. and has announced multiple clinical trials, including FDA clearance of several Investigational New Drug (IND) applications using Ossium products. It has also partnered with the National Marrow Donation Program (“NMDP”), which runs the world’s largest living donor registry, to help bring its bone marrow to patients looking for a match. It has received funding from several different U.S. science agencies as well as some of the most highly regarded venture capital firms in the world (notable investors include First Round Capital, General Catalyst, Vivo Capital, and Village Global).
Dr. Brandacher, Dr. Sachs, and Seb have left the company, but in his professorship Dr. Brandacher kept a research collaboration with Ossium. The resulting peer-reviewed publications give some indication of what the bone marrow bank might be capable of achieving in the future. Among the new discoveries are a novel use of the bone marrow bank as a source of mesenchymal stem cells (“MSCs”). MSCs have known or suspected potential therapeutic value in an enormous range of conditions beyond those that the bone marrow bank was originally intended to treat: heart attacks, Alzheimer’s and other neurodegenerative diseases, autoimmune conditions, and liver and kidney failure, to name a few. Dr. Brandacher and Ossium researchers have reported that MSCs can be collected from the tissue recovered by Ossium at orders of magnitude higher abundance than traditional sources.
With roughly half of the U.S. OPOs now partnered with Ossium to facilitate donation of this “ninth donor organ” and top investors funding the effort, the lifesaving potential of each organ donor seems to be on a path to grow much larger. A single donor could turn out to be capable of saving or improving dozens of patients’ lives than was previously thought …maybe even delivering human health impact on par with all other organ transplants put together.
Fast-Tracking Solutions to the Organ Shortage
In the earliest days of OPA, Seb and his fellow volunteers visited the laboratory of Professor Mehmet Toner at Massachusetts General Hospital (“Mass General”). Mass General is the flagship hospital of the Harvard Medical School system and a biomedical research giant in its own right. Mehmet breathes rarified air even among professors there, publishing regularly in top tier journals such as Nature and Science. One of his greatest sources of fame is as a pioneer in early cancer detection. In 2011 Johnson & Johnson gave Mehmet and his collaborators $30 million to develop a liquid biopsy technology that they had invented to diagnose cancer in blood samples. Mehmet and his colleagues used this to run an industry-style R&D program within the research hospital, ultimately spinning out multiple biotech companies. Over the decades prior to OPA, Mehmet had also helped keep the field of tissue cryopreservation moving forward – during an era when there was little funding available in the field and few serious scientists deemed it interesting or tractable enough to work on. Many of today’s leading cryopreservation researchers started their careers in the Toner Lab.
Mehmet and his protégé at Mass General, Professor Korkut Uygun, were working on an intriguing approach to organ cryopreservation. Rather than contend with the full challenge of reaching deep cryogenic temperatures (below -140℃) to enable unlimited organ storage durations, Uygun and Toner developed methods to safely cool the organ to relatively “high” below-freezing temperatures. In 2014 they cooled rat livers to -6℃ and stored them for 3 days before transplanting them. The procedure worked in 100% of the rats, and the results were published in Nature Medicine.
In large part this works for transplant organs for the same reasons it works for the food in your refrigerator: cold slows molecular motion and lowers the rates of chemical reactions. Lowering the temperature extends the window of time before irreversible injury starts to occur and the organ starts to deteriorate. For practical purposes, deep cryogenic temperatures (below about -140℃) arrest all molecular motion within the organ – tantamount to having “stopped” biological time. But Toner and Uygun had set an easier target: making biological time move more slowly than it ever had before, in a safe and controlled fashion.
Mehmet wanted to know if Seb would be willing to spin out a biotech company to commercialize the research. This was no small ask: Seb was already working to build a bone marrow bank from scratch, and the future of the fledgling OPA (at this point still an informal advocacy group) was far from certain. On the other hand, this “high sub-freezing temperature” approach could be exactly what Seb had been looking for: the fastest scientific path to remove at least most of the transplant system’s Achilles Heel. Even preservation times of a few days would vastly expand the options to transplant each donor organ. If this research could be accelerated by even a few years, it could mean saving many thousands of patients’ lives.
It turned out that another renowned cryopreservation researcher had been circling the same solution: Dr. Michael Taylor (“Mike” for short). In the field of cryobiology, Mike was one of the few scientists working on the deep cryogenic storage of living tissues even before OPA’s rise. Over a decade before, Mike and another well-known researcher in the field, Dr. Kelvin Brockbank, had made a critical cryopreservation breakthrough: they had demonstrated the first vitrification (ice-free cryopreservation) of a large solid tissue with function and transplant success after storage at deep cryogenic temperatures (below -140℃), opening up the possibility of larger living systems like transplant organs and helping lay the foundation for the research effort later catalyzed by OPA. The results were published in the preeminent bioengineering journal Nature Biotechnology. But well before helping uncover how organs and tissues can survive the deep freeze, Mike had experimented with approaches to safely bring organs to the same “high” below-freezing temperatures that the Mass General researchers now had in their sights.
In the field of transplantation Mike was best known as one of the developers of the LifePort Kidney Transporter. For many years the LifePort had been the market-leading device to preserve kidneys while they were transported from donors to recipients. It was the first of a new generation of devices to perfuse donor organs prior to transplantation: while traditionally transplant organs were simply placed on a bed of ice and rushed from the donor to the recipient, the LifePort provided a sort of artificial circulatory system. It supplied a blood substitute to the organ’s own vasculature, filled with medicines and preservatives to keep the organ healthy throughout its journey.
The LifePort had kicked off a seismic shift in organ transplantation that is still unfolding today. Increasingly transplant hearts, lungs, livers, and kidneys are perfused after recovery from the donor’s body in order to mimic the organ’s natural environment. This allows OPOs and transplant teams to collect data on the organ’s health and function, better predicting how well it might perform in the recipient’s body. Many different devices are now marketed or in clinical trials. These have played a significant part in the dramatic rise in organ transplants over the last decade: they have been widely adopted in Europe, and adoption has also steadily increased in the U.S. despite the country’s slower regulatory approval process.
These platforms are popular largely because they can resolve many concerns that a transplant team might have about whether the organ will function well enough to be worthy of transplanting into their patient. Whereas increased preservation times can allow organs to be offered to more centers, organ perfusion can allow more of those centers to say “Yes” to each offer.
The perfusion platforms developed by Mike and others have also helped open the door to organ cryopreservation. They can be used to deliver all of the preservatives involved in the cryopreservation process. They can rapidly cool and warm organs using its own circulatory system. And they can allow cryopreservation researchers to efficiently assess the organs’ function before and after cryopreservation experiments – even for human organs, prior to any clinical trials. The widening arsenal of organ perfusion devices could also be easily integrated with cryopreservation technologies; cryopreservation itself could be performed using these devices, constituting nothing more than an additional step in the organ’s journey from donor to recipient.
Along with Dr. Toner at Mass General, Seb and Mike formed the public benefit company Sylvatica Biotech. To complete the founding team they later recruited Dr. Uygun, as well as three other scientists. The first was Dr. Brad Weegman, a young postdoctoral researcher working in the field of tissue preservation who had produced an exceptional output of high-quality research publications in his short career. The second was Dr. Simona Baicu, who had managed the surgical recovery of donor tissues at a major OPO before working with Mike to develop the LifePort organ perfusion device. The final Sylvatica co-founder was Dr. Shannon Tessier, a postdoctoral fellow in the Toner Lab and now an Assistant Professor at Harvard Medical School and Mass General.
Dr. Tessier had come to Mehmet’s lab after studying under another renowned cryopreservation researcher: Professor Ken Storey at Carleton University in Ottawa, Canada. Dr. Storey had published a massive body of research (more than 1,200 research articles) on the adaptations that allow some arctic species to survive below-freezing temperatures in a state of torpor (suspended animation). One of the most famous research subjects in the Storey Lab was Sylvatica Biotech’s namesake, Rana Sylvatica: the arctic wood frog.
Every winter this remarkable little frog prepares its body for the cold with a highly orchestrated “shut-down” procedure. Its tissues release chemicals that protect it from the harmful effects of ice and extreme cold. Its metabolism is altered to eliminate the side-effects of sudden interruption of its oxygen supply, the primary source of harm to transplant organs outside the body today. After this process, the extreme Canadian winter can expose the frog to temperatures below -20℃. Most of its blood freezes, and it has no heartbeat or brain activity. Yet when spring returns and the frog’s surroundings warm back above the freezing point of water, it resumes normal life. Most remarkable is the apparent lack of adverse side effects, aside from hunger …and an eagerness to mate.
These kinds of cold-tolerant adaptations are found throughout the animal kingdom, including in mammals. With the rise of genomics and other ‘omics technologies it had become possible to map these adaptations in molecular detail, and maybe even transfer them from arctic species to other species from warmer climates – including humans.
The Sylvatica researchers reasoned that it should be possible to use engineering approaches to give transplant organs the same protection found in the genes of Rana Sylvatica and other species. And like many other Nature-inspired approaches in modern medicine, they could engineer these advantages to be even more effective and efficient than what Nature had managed to accomplish by chance.
To achieve this Sylvatica launched multiple research programs in parallel. Since then it has won numerous government research awards. In 2020 the National Institutes of Health, the main funder of biomedical research in the U.S., featured Sylvatica as one of the agency’s success stories. While much of the company’s R&D is still under wraps, some of the work with the Harvard and Mass General team can be tracked through publications in prestigious journals such as Nature Biotechnology, Nature Communications, and Nature Protocols, which have been widely covered by media outlets such as The Atlantic, scientific journals such as Science, and medical journals such as the Journal of the American Medical Association (JAMA).
With the technology now pushing toward several days of preservation time in human livers, Sylvatica is working with a growing number of OPOs and transplant surgeons to move its research program into the clinic. The company seems poised to deliver on its original promise: overcoming the Achilles Heel of the U.S. transplant system by allowing OPOs to offer organs to transplant programs across a much greater time and distance.
Dr. Sebastian Eriksson Giwa
Q&A With Dr. Sebastian Giwa on the Future of Cryopreservation
I asked Seb Giwa about how the impact he sees unfolding matches up to his original vision over a decade ago on the NASA campus. Seb and I agreed that the sheer scope of this question calls for another article and a more in-depth interview (stay tuned!). But he offered some very provocative thoughts for this article.
Alex: What’s something we haven’t covered that excites you about what cryopreservation can do for the organ shortage?
Seb: In the long-term, one of the things that I’m most excited about is how these technologies can remove barriers to developing transplant systems in new countries. Most of the world still doesn’t have access to deceased donor transplantation. For instance, Africa has 16% of the world’s population but only 0.5% of transplants are done there. Meanwhile the U.S. has less than 5% of the world’s population but does about 25% of the world’s transplants. Many developed countries, like my father’s home country Ghana, have limited live kidney donation programs. But they don’t have deceased donor programs, which are needed to carry out large numbers of kidney and liver transplants as well as any sort of heart or lung transplantation.
That’s partly because the logistical demands to source organ donations prospectively require so much infrastructure: a waitlist, rapid matching of donors to recipients, OPOs that need to be overstaffed in order to deal with unpredictable surges in organs available, rushed activities that require tight coordination between donor hospitals, OPOs, transplant centers, and even third party service providers like organ couriers. Many organs have also needed expensive transportation (private jets and helicopters).
It’s a very different situation when there’s a source of cryopreserved hearts, livers, etc., that can be donated in a much more flexible way and are simply waiting to be matched to patients. Even things like Doctors without Borders and perhaps “OPOs without Borders” become possible for transplantation, helping train and develop new organ recovery, heart, lung, and liver transplant programs. So many more possibilities open up when you don’t need to create every part of a transplant system from scratch and have all of those parts acting in synchrony on Day 1.
In some ways organ transplantation would be even more impactful in developing countries. For instance, patients in the U.S. and other developed countries can live for a very long time with the aid of dialysis, whereas people in developing countries don’t have access to it. Without kidney transplantation, renal disease can become a death sentence much more quickly in those places.
In many developing countries, causes of death that make someone a candidate for donation are also more common: traffic accidents, construction accidents, and so on. Because of demographic differences these deaths happen more commonly in younger populations than in developed countries. The organ donors are there in principle. Through cryopreservation we can ease the process of developing transplant systems to make those donations possible.
Over time, this might increase lifesaving transplantation globally by 10- or 20-fold. It’s a massive opportunity to help give the gift of life.
Alex: How about things outside the organ transplantation space? Can you give us a teaser for the future Forbes.com article?
Seb: There are a lot of things that I’m excited about here, but I’ll make a plug for one that has massive untapped potential. I’ve been working on this with Jedd Lewis and Dr. Gloria Elliott at Organ Preservation Alliance (CEO and Chief Science Officer there, respectively).
Today, cryopreservation can unlock an abundant source of functional human donor brain tissue that could be transformative for research on neurodegenerative and neurological diseases. Over the last decade research on functional brain tissue has undergone a step change: human brain slices can now be cultured for weeks in the lab. These tissue slices are not “alive” in the sense that they can’t think and feel like the donor did, but the cells inside the tissue remain functional and the “biological ecosystem” is intact.
OPA is also working on this concept using pancreatic tissue for Diabetes research and other tissues that have undergone a similar leap forward in laboratory culture capabilities. But I’ll focus on brain tissue here because there’s such an acute need for human data sources in brain diseases like Alzheimer’s and Parkinsons. It’s a major gap in medical science, and neuroscience research programs like the Allen Institute for Brain Science have been focused on figuring out how to fill that gap using “living” donor brain tissue.
A thin slice of tissue might not sound like much to the casual reader, but just having this intact, functioning human tissue environment – with the donor’s cells still working together the same way they do in the brain – is a massive leap forward from what’s available in brain disease research today.
Nearly all new medicines for neurodegenerative diseases fail when they’re first tested in humans, after years being tested on animals. On average those failures can cost more than $100 million out of pocket, putting the cost per successful treatment in the billions. That’s partly because animal brains are so different from human brains, and it’s also because the animals themselves don’t have the diseases that we’re trying to cure.
This functional human brain tissue can offer something new and profound to drug discovery and medicine. Not just because it’s human, but because it retains all the changes that the tissue has undergone (and is still undergoing) as a result of the real human donor’s real disease. It’s the difference between studying a very, very rough imitation of a disease (in animals) and studying large parts of the real disease from a real patient.
There are many potential brain donors among the patients referred by donor hospitals to OPOs. In theory each donor has enough brain tissue to provide hundreds of thousands of tissue samples. This means that orders of magnitude more donor brain tissue could come from this source than is possible with the surgical resections that scientists are forced to rely on today. New methods need to be developed, but all of the fundamental technologies already exist to procure and study all of this brain tissue.
What’s been missing until recently has been cryopreservation. For most disease research applications it’s prohibitively impractical to receive an entire (whole) brain donation and isolate tissue, just to do a few experiments on one donor before the tissue expires. Jedd has a good analogy for this: imagine if every time a drug hunter like yourself needed to do an animal study, you had to go out into the woods and catch a new mouse. It would be terribly slow and expensive, and the sample size you could achieve would often be too small to overcome the genetic diversity of the animals. (In the 1920s researchers started using in-bred mouse strains, which are essentially genetically identical.) Most of what we take for granted in medical research wouldn’t have been possible. The situation has been the same for research using human tissue, until now.
With cryopreservation all of the tissue can be built up in a bank ahead of time. Tissue from the same donor can be used again and again, allowing you to cleanly interpret data because you’re comparing results within the same donor. And because a single donor can provide tissue for thousands of these experiments, when a tissue banking operation is fully scaled the tissue might actually become less expensive to procure than the containers the tissue is stored in. There’s always more tissue there when the researchers need it – even without notice, and even for for iterative experiments (like lead optimization in drug discovery) that need to happen day after day after day.
Banks of hundreds of donors can be built up over time. This means that when needed, these kinds of on-demand experiments can actually be the size of clinical trials. The donor populations for some diseases can be as diverse as the patient populations, allowing us to unravel disease sub-types and build precision medicine into our treatment hypotheses from the beginning of a drug discovery program.
In a very important way, human studies using the brain tissue bank can actually capture much more diversity than clinical trials today. We can ensure gender balance and ethnic representation in a way that hasn’t been possible with clinical trial enrollment. Today’s medicines are typically evaluated based on how safe and effective they are for white males. Using a bank of functional tissue, equity is built into drug discovery from the very beginning of the process.
We can also do something else that isn’t possible with clinical trials today. Remember that for practical purposes, cryopreservation is the same as stopping biological time. Because all of the tissue from a given donor is cryopreserved at the same time, we can test potential medicines on a human donor’s tissue, collect data… and then “go back in time” to test the next iterations of the medicine on the same human donor. It’s like Groundhog Day for human disease research.
Right now this opportunity is similar to where Ossium was when I started it in 2015. In this case there are certainly commercial spinout opportunities, but also a lot of foundational (non-profit) research that needs to be done so that donor hospitals across the world can be banking functional brain tissue in this way. OPA’s Biostasis Research Institute has funding for some initial work that’s been generously provided by OPOs and other donors.
That’s a good start, but to move this forward we need talented neuroscientists, drug discovery companies, organ recovery technicians, clinicians, more OPOs and organ recovery partners, and more funding. Over time: a lot more funding.
Alex: That’s an amazing vision Seb. Let’s definitely dive more deeply into it in the next issue. For now, any closing message for our readers?
Seb: These are big topics and can be a bit abstract, so it’s easy to lose sight of the real people that all of these OPOs, transplant centers, and researchers are trying to help. One thing that impressed me early on is how deeply that awareness is ingrained in the organ donation community. When you visit most OPOs you’ll see pictures of organ donors lining the walls. Recipients too… Their battle doesn’t end when they get the new organ.
The OPO staff place a lot of emphasis on honoring these donors and recipients. They memorialize them. It’s similar to what you see in other contexts for veterans and service members: showing gratitude to those who have fought and are still fighting, and honoring the fallen. I don’t think I’ve ever met someone leading an OPO who isn’t completely genuine about the mission of honoring each donor’s lifesaving gift.
So I’ll try to do the same here by honoring a friend of mine, and a tremendous advocate for transplantation, who passed away recently: Charity Tillemann-Dick.
Charity was a professional opera singer who performed at places like the Lincoln Center and Carnegie Hall and performed for world leaders – before and after receiving a lung transplant for pulmonary hypertension. When I met her in the early days of OPA, she had already had two double-lung transplants. She died a few years later at the age of 35, from cancer caused by the immune suppression drugs that she took to keep her body from rejecting the donor lungs.
You can watch a talk that Charity and I gave together, and watch her TEDMED talk. Charity deserves recognition for many things that she did as a transplant advocate, and one of them was helping get the organ cryopreservation research effort off the ground. Charity spoke to scientists and research funders at OPA’s Organ Banking Summits, at a time when most weren’t working on organ cryopreservation and were deciding how much to get involved. She performed there. They were blown away that she could still sing like that, on her third set of lungs. It was the highlight of those events. And it really showed the power of transplantation, what a miracle it is.
We’re now in a world where these technologies can bring that miracle to a lot more people – and potentially for lifelong durations. I’ll always be grateful to Charity for helping create that world.
Register to Be an Organ Donor
To register as a bone marrow donor, click here. To register as an organ, eye or tissue donor, click here. Both processes are easy and only take a minute.
The author would like to thank Dr. Sebastian Eriksson Giwa and Jedd Lewis for their time, materials, and valuable contributions to this article to to the rapidly developing field of cryobiology.