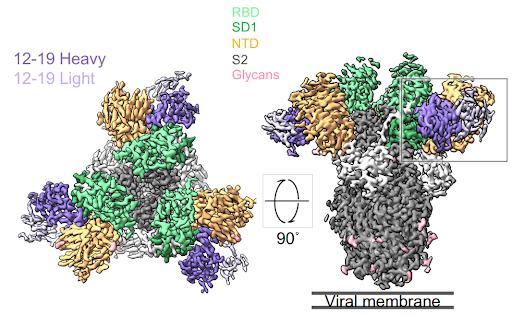
12-19 antibody binding across Spike subunits
A new antibody pair neutralizes all current variants of concern of SARS-CoV-2. There is an effective need for monoclonal antibody treatments for those suffering from moderate to severe Covid-19. While a number of treatments have been developed throughout the pandemic, the virus is continuously mutating, rendering many of these treatments obsolete. The key is to find antibodies that target conserved regions of the virus, allowing the antibody to neutralize a broad range of variants.
Two such broadly neutralizing antibodies were recently identified by Liu et al. Many monoclonal antibodies target the spike receptor-binding domain, the region that makes contact with the host cell’s ACE2 receptor, triggering infection. The two antibodies defined by Liu et al. target different regions, the N-terminal domain, SD1, and the S2 region. Here we analyze these antibodies and discuss how their addition impacts the current monoclonal antibody treatments pool.
Isolation and characterization of broadly neutralizing monoclonal antibodies against SARS-CoV-2 variants
The researchers evaluated a panel of sera samples against a range of 11 SARS-CoV-2 variants in search of neutralizing activity. Sera from patient 12 demonstrated the highest levels of neutralization. From patient 12’s sera, they isolated 27 monoclonal antibodies, of which five neutralized Omicron BA.1. From these five, antibody candidates 12-16 and 12-19 demonstrated the strongest neutralization.
Both 12-16 and 12-19 further neutralized some of the latest and most prevalent variants of SARS-CoV-2, including BQ.1.1, XBB.1.5, and CH.1.1, all of which have highly mutated spike proteins.
In hamster models, 12-16 and 12-19 significantly reduced virus titers in subjects infected with Omicron BA.1, reducing the virus to undetectable levels within days.
Antibodies 12-16 and 12-19 target a quaternary epitope between NTD and SD1
Liu et al. used cryo-electron microscopy to determine the binding epitope of 12-16 and 12-19 to the SARS-CoV-2 spike protein. Rather than binding the typical receptor-binding domain, the antibodies bound the N-terminal domain, subdomain 1, and a small section of the S2 region.
FIGURE 1: Cryo-EM reconstructions of antibodies in complex with SARS-CoV-2.
Most antibodies throughout the pandemic bound the receptor-binding domain from residues 333 to 527. The N-terminal domain runs from residues 14 to 305, subdomains 1 and 2 run from residues 541 to 685, and S2 is the latter half of the virus from residues 685 to 1273.
12-16 and 12-19 bind the spike at slightly different angles. 12-16 binds at a higher angle, favoring the N-terminal domain, whereas 12-19 binds at a lower angle, binding more sites on SD1. From here, we will focus on 12-19 as it binds more completely in vitro than 12-16, likely due to its slightly more cross-region epitope.
12-19 neutralize SARS-CoV-2 by locking RBD in the down conformation
Akin to some antibodies seen throughout the pandemic, 12-19 blocks infection by locking the receptor-binding domain in the down conformation. Before contact with the host cell, the spike shifts from a down to an up conformation, allowing binding and enabling infection of the cell. Infection is prevented if the receptor-binding domain is locked in the down conformation.
But how can an antibody that binds the N-terminal domain, subdomain 1, and S2 impact the receptor-binding domain? Part of the 12-19 epitope binds residues that are shifted during conformation change, namely in subdomain one and a linker region between the N-terminal and receptor-binding domains. Imagine two gears in the same link. They may not necessarily be consecutive, but block the movement of one, and the whole line of gears is stalled.
FIGURE 2: The 12-16 and 12-19 antibody structures (colored) were superimposed onto an apo 500 … [+]
By blocking the conformational shift, 12-19 impedes ACE2 binding between the receptor-binding domain and the host cell.
Another set of antibodies that uses conformation locking is the camelid nanobodies described in 2021. These bind across receptor-binding domains of a spike trimer, preventing the spring-loaded conformation switch from engaging.
FIGURE 3: Cryo-EM reconstruction of camelid antibodies in complex with trimeric SARS-CoV-2 spike.
The epitope of antibody 12-19 is highly conserved
As with any monoclonal antibody candidate, we must consider the potential escape mutations enabling a viral variant to escape neutralization from 12-19. Using deep mutational scanning libraries, Liu et al. found a few potential problem residues.
The areas most likely to produce escape mutations were the N4 loop of the N-terminal domain from residues 172-176, deletions throughout the N-terminal domain loops, namely residues 103 and 121, as well as residues 522, 561, and 577 in the subdomain 1.
However, these residues are rarely mutated in currently circulating variants. The most frequent is L176F, found in only 0.2% of sequenced variants. This indicates that the epitope of 12-19 is largely conserved, at least so far in the pandemic.
Discussion
On first inspection, 12-19 would serve as a valuable antibody treatment, given its strong neutralization of the latest SARS-CoV-2 variants. An advantage it may have over other treatments is its unique binding epitope. The figure below compares the binding epitope of 12-19 to other neutralizing antibody epitopes from throughout the pandemic.
Liu et al.
FIGURE 4: (A) SARS-CoV-2 spike protein with distinct subregions outlined schematically by color. (B) … [+]
Compounding the unique binding epitope of 12-19 is it’s reaching across spike domains to lock conformation. We believe conformation locking is among the most potent mechanisms by which an antibody can neutralize a virus.
The 12-19 antibody is not our first encounter with conformation-locking antibodies. In fact, some of our most antibody candidates to other dangerous pathogens employ a similar method. Lassa virus antibody 8.9F binds across the three faces of the Lassa trimer, locking the glycoproteins into place. Ebola antibodies 1C3 and 1C11 use a similar mechanism, locking the whole of the Ebola structure in place. Even parasites such as Malaria can be overcome with conformation-locking antibodies, such as CIS43, which prevents a cleavage function required for malaria infection.
The need for effective monoclonal antibody treatments is urgent. We should constantly look for antibodies that employ this conformation-locking tactic against SARS-CoV-2 and other major pathogens. These treatments consistently provide strong neutralization and protection. Their addition to the antibody arsenal would be well worth the effort to find them.