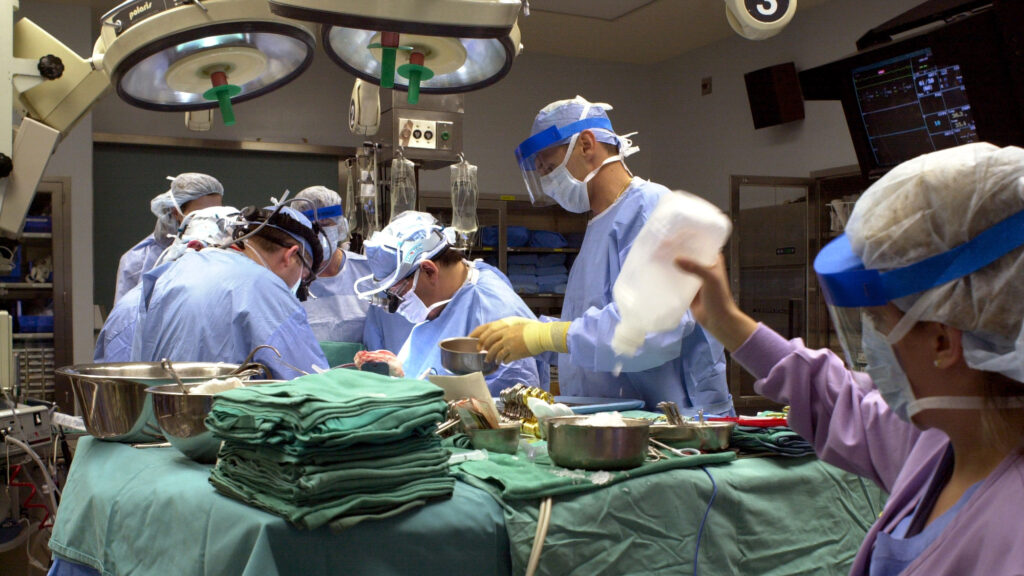
A new method of heart transplantation that uses machines to reanimate donor hearts from people who have died is just as good as traditional heart transplantation, a new study finds. If adopted broadly in the U.S., the procedure that could expand the donor pool by 30%.
The adjusted six-month survival rate of patients undergoing the new method was 94%, compared with 91% among patients who underwent the traditional method, according to the study published in the New England Journal of Medicine Wednesday, the first large randomized study comparing the two procedures.
At one year, the survival rate was 93% for patients who got the reanimated hearts, versus 85% for those who got hearts under the traditional procedure, according to the study, which was funded by TransMedics, the company that makes the heart machine.
Doctors in Australia and the U.K. were first to start using this new method. U.S. surgeons later performed the first one in the country in 2019. While usage of the procedure has been increasing since then, it’s still used in just a small minority of cases, making up about 6% of all heart transplants in the U.S. in 2021.
“Hopefully this study shows people that this is an equivalent treatment and should be standard of care for all recipients,” said Jacob Schroder, first author and a heart transplant surgeon at Duke University. “As a medical community, we should understand that and get over this historical thought that this can’t be done.”
The traditional method of transplantation involves taking hearts from donors who are brain-dead, and then immediately putting the hearts in cold storage to transport.
The new method uses donations after circulatory death (DCD) from people who have suffered major neurologic injury and are on life support, but don’t meet the strict definition of brain death. Once there’s consent, life support is withdrawn and the transplant team waits until the donor has died. Then, the team goes in, takes the heart into a machine that circulates blood through it and keeps it functional through transport.
Jacob Schroder
The study included 180 participants, with 90 patients in each group. Not only did patients in the DCD group have equivalent — if not better — survival outcomes, they also received their transplants slightly faster, waiting on average 24 days compared with 31 days for patients in the traditional group.
Schroder noted that while the DCD method is costly to perform due to the machine, it may also yield cost savings if patients are spending less time in the hospital before they get their transplant.
Patients in the DCD group, however, did have higher rates of dysfunction with their transplanted heart — 22% compared with 10% of those in the traditional group. None of the patients in the DCD group had to undergo retransplantation, though, while two people in the traditional group did.
Maziar Khorsandi, a cardiothoracic surgeon at the University of Washington who performed the first DCD heart transplant in the Pacific Northwest, said “the results are pretty reassuring.”
Ever since his center started doing these procedures about a year ago, their caseload has increased about 20%, he said. It’s difficult for a center to start using this method since the machines are costly and transplant teams have to be trained on using them, but still, the method has “revolutionized what we do and how organs can be more available for patients who are suffering from heart failure and actively listed on the waiting list.”
STAT’s coverage of chronic health issues is supported by a grant from Bloomberg Philanthropies. Our financial supporters are not involved in any decisions about our journalism.