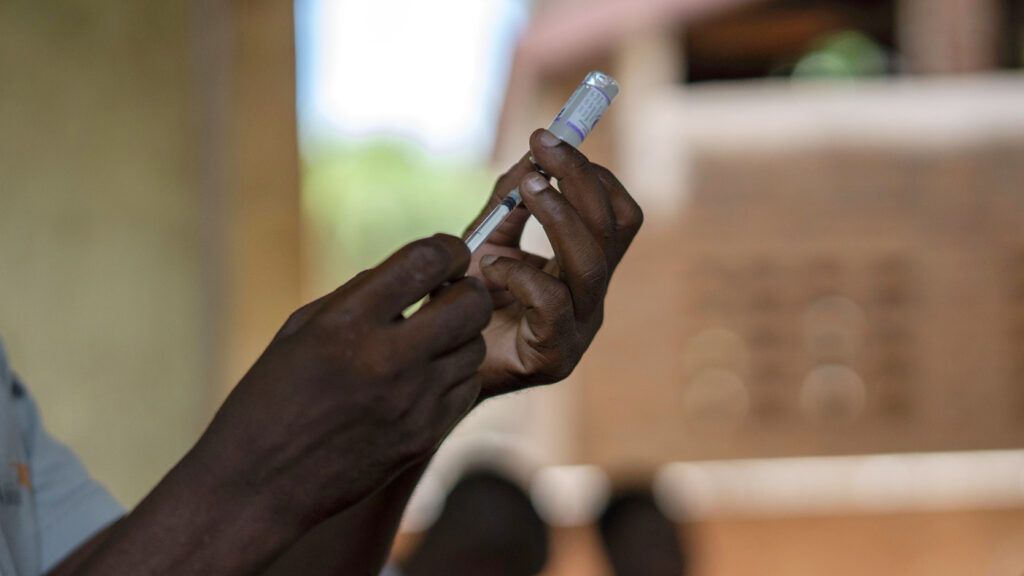
Just two years after the World Health Organization’s historic recommendation of the first malaria vaccine, the global health agency on Monday recommended a second, seeking to broaden access to a tool it hopes can save lives.
The vaccine, called R21/Matrix-M, was developed by the University of Oxford and will be produced by the Serum Institute of India, the world’s largest vaccine manufacturer.
The WHO’s Strategic Advisory Group of Experts on Immunization (SAGE) and the Malaria Policy Advisory Group (MPAG) found no significant difference between the efficacy of the two vaccines. When delivered in a similar way — and administered at first right before the high-transmission period of the disease — both multi-dose vaccines were found to have an efficacy of about 75%, WHO’s senior technical officer, Mary Hamel, said at a press conference.
Malaria causes an estimated 247 million cases each year, including among 25 million children in areas of moderate and high-transmission risk. In 2021, the disease killed 619,000 people, according to the WHO, overwhelmingly in sub-Saharan Africa and among children, who make up 80% of the deaths.
“As a malaria researcher, I used to dream of the day we would have a safe and effective vaccine against malaria. Now we have two,” WHO Director-General Tedros Adhanom Ghebreyesus said during the press conference.
The new vaccine should help to address supply constraints, WHO officials said.
The vaccine recommended by the WHO in 2021 is known as RTS,S — its brand name is Mosquirix — and is marketed by GSK. The company has agreed to supply 18 million doses of vaccine to 12 African countries between 2023 and 2025, and is planning to deliver 15 million annually between 2026 and 2029, according to a company spokesperson.
The Serum Institute of India, however, is already able to deliver a supply of much larger magnitude: It already has plans in place to produce 100 million doses of Mosquirix a year, and plans to scale up to 200 million, providing much-needed supply to meet the demand.
Mosquirix vaccination protocol requires three doses at monthly intervals for the primary series, followed by a booster 18 months after the last injection. It is recommended for children aged six to 17 months at the time of the first dose.
While both vaccines have a similar efficacy profile, there are other factors that may determine whether a country prefers one over the other, in particular pricing and the delivery methodology.
GSK’s vaccine is sold at cost, capped at €6.90 per dose (about $7.25) even if the cost of production should increase, according to the procurement agreement with UNICEF. The company also agreed to revise the price downward should its production costs decrease. (GSK said it believes that the cost of production will decrease, in part because of plans to have India’s Bharat Biotech take over production in 2029.)
Doses of R21/Matrix-M, however, are cheaper — between $2 and $4. SAGE recommended four doses in children aged five months and up, with a fifth dose recommended for children living in areas of high risk. This adds up to about half the price of the GSK vaccine.
Most countries will receive the vaccine at a subsidized cost through GAVI, the Vaccine Alliance, so “the differentiation in price is most significant for those countries which are soonest to leave GAVI’s support,” said O’Brien.
Another significant difference, said O’Brien, is there is ample body of safety data on GSK’s vaccine, while it will take time for further safety monitoring to collect comparable data for R21/Matrix-M.
Overall, whether a country administers one or the other vaccine may be based on availability.
So far 18 countries have applied to receive the vaccine through GAVI, and the WHO says it expects to be out of any shortages by the third quarter of 2024. However, the expectation is that more countries will apply to receive the vaccine now that more will be available.
“Definitely the demand will be high,” said Daniel Madandi, the director of the Global Malaria Program.