Topline
The Food and Drug Administration approved Opill, the first over-the-counter oral birth control pill, and because it’s a type of birth control called a mini pill, it’s safer for some patients than alternatives that have higher risks of blood clots and developing breast and cervical cancer.
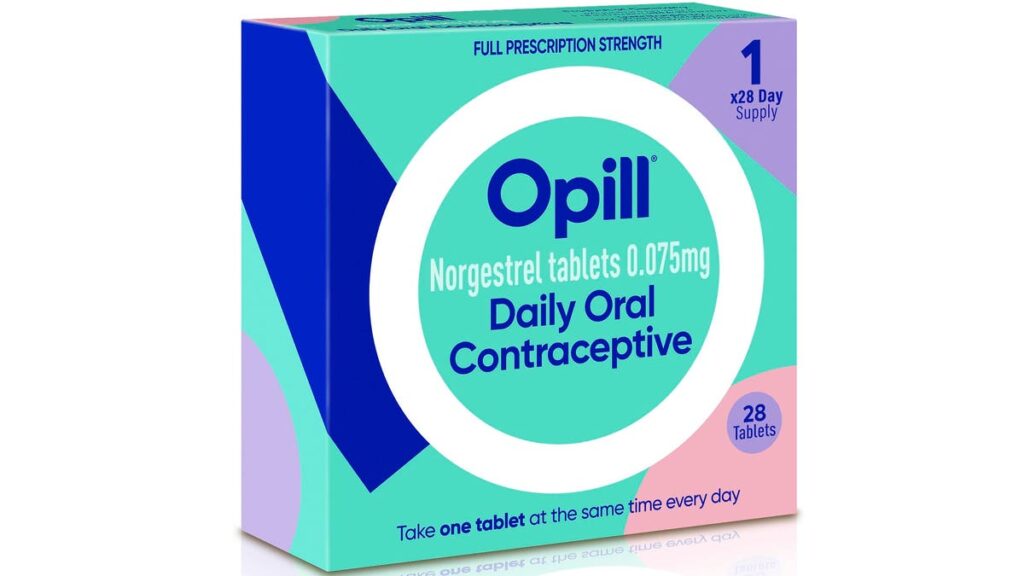
This illustration depicts proposed packaging for the company’s birth control medication Opill.
Key Facts
Opill is drug maker HRA Pharma’s brand name for once-daily norgestrel, an oral contraceptive that contains the female hormone progestin and is used to prevent pregnancy.
Combination birth control pills (like the brands Loestrin, Nordette and TriNessa) and mini pills (like Errin, Jolivette and Heather) are already available on the market, but they require a prescription from a medical professional to use.
Norgestrel was first approved by the FDA in 1973 for prescription-only use under the brand name Orvette, which was discontinued in 2005—not due to safety concerns—and the name change to Opill was approved in 2017.
Key Background
Norgestrel is a progestin-only birth control pill that works by thickening cervical mucus, which stops sperm from penetrating, thus preventing ovulation, the FDA reports. It also slows down the egg’s progress through the fallopian tubes. To be effective, the pill must be taken at the same time every day, and taking medicines that interfere with its efficacy—like antibiotics—may increase the risk of pregnancy. Common side effects include weight gain or loss, change in sex drive, facial hair growth, fluid retention, change in menstrual flow and irritability. However, patients should seek medical attention if they experience a handful of other side-effects, including: breast tenderness or discharge, unusual tiredness, yellow skin or eyes, severe headaches, shortness of breath, speech or vision problems, pain in the abdomen, legs, groin or chest and itching, skin rash or hives.
Tangent
There are two main types of birth control pills: combination pills and mini pills. Combination pills contain both progestin and estrogen, another female hormone. Norgestrel is a mini pill because it only contains progestin. Combination pills come in a variety of different forms, with some containing between 21 and 24 active pills and seven to four inactive pills, and others containing 84 active pills and only seven inactive pills. Bleeding, or menstrual periods, happens when patients on the combination pills take the inactive pills, allowing patients to decide how often they want their periods. Mini pills, on the other hand, contain all active pills, though menstrual cycles still occur. Because they don’t contain estrogen, which increases risk of blood clotting and cervical and breast cancer, mini pills are recommended for people with high blood pressure, a history of blood clotting and a high risk of heart disease, as well as those who recently gave birth, are older than 35 and smoke and have a history of migraines. However, mini pills provide less leeway, as a pill is considered missed if taken more than three hours late.
Big Number
46.9 million. That’s how many American women between the ages of 15 and 49 used some form of contraceptive between 2015 and 2017, according to the Centers for Disease Control and Prevention. That’s about 65% of the 72 million women in that age demographic.
Crucial Quote
“Today’s approval marks the first time a nonprescription daily oral contraceptive will be an available option for millions of people in the United States,” Patrizia Cavazzoni, director of the FDA’s Center for Drug Evaluation and Research, said in a statement. “When used as directed, daily oral contraception is safe and is expected to be more effective than currently available nonprescription contraceptive methods in preventing unintended pregnancy.”
Further Reading
FDA Approves 1st Over-The-Counter Birth Control Pill (Forbes)